Learning Outcomes:
i. Define free radical substitution and explain its significance in organic chemistry.
ii. Describe the mechanism of free radical substitution in alkanes, including initiation, propagation, and termination steps.
iii. Analyze the factors affecting the selectivity of free radical substitution reactions.
iv. Predict the products of free radical substitution reactions in alkanes.
v. Appreciate the role of free radical substitution in various industrial applications.
Introduction
Free radical substitution is a fundamental reaction mechanism in organic chemistry, particularly for saturated hydrocarbons like alkanes. This lesson delves into the intricate mechanism of free radical substitution in alkanes, providing insights into how these hydrocarbons undergo substitution reactions in the presence of free radicals.
i. The Essence of Free Radical Substitution
Free radical substitution involves the replacement of a hydrogen atom in an alkane with a different group, such as a halogen atom. This reaction occurs through a chain mechanism involving three key steps: initiation, propagation, and termination.
Initiation: The reaction begins with the formation of free radicals, typically initiated by heat, light, or the presence of initiators. For instance, chlorine gas (Cl2) can be broken into chlorine radicals (Cl•) under ultraviolet (UV) light.
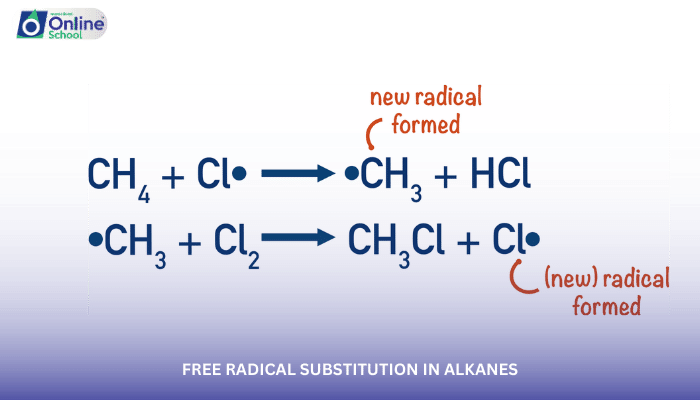
Propagation: Free radicals act as reactive intermediates, attacking alkane molecules and abstracting hydrogen atoms to form new alkyl radicals and hydrogen halides (HX). The alkyl radicals, in turn, react with halogen molecules, leading to the formation of halogenated alkanes and new halogen radicals, perpetuating the chain reaction.
Example:
Cl• + CH4 → CH3• + HCl
CH3• + Cl2 → CH3Cl + Cl•
Termination: The chain reaction eventually terminates when two free radicals combine to form a stable molecule, such as a halogenated alkane or a dimer.
ii. Factors Affecting Selectivity
The selectivity of free radical substitution reactions is influenced by several factors, including:
Bond Strength: The strength of the C-H bond in different positions of an alkane varies. Tertiary carbon atoms (C-H) have weaker bonds compared to primary or secondary carbon atoms, making them more susceptible to attack by free radicals, leading to preferential substitution at tertiary positions.
Steric Hindrance: Bulky substituents on an alkane can hinder the approach of free radicals to specific carbon atoms, affecting the selectivity of the reaction.
iii. Predicting Products of Free Radical Substitution
By understanding the mechanism and factors affecting selectivity, chemists can predict the products of free radical substitution reactions in alkanes:
Monosubstitution: Free radical substitution typically results in the formation of a mixture of monosubstituted products, with the relative amounts of each product depending on the factors mentioned above.
Regioselectivity: The preference for substitution at tertiary carbon atoms leads to regioselectivity, where tertiary halides are formed as major products compared to primary or secondary halides.
iv. Industrial Applications
Free radical substitution reactions play a crucial role in various industrial applications:
Halogenation of Alkanes: The production of halogenated alkanes, such as chloromethane (CH3Cl) and dichloroethane (C2H4Cl2), used as refrigerants, solvents, and precursors for other chemicals.
Alkylation: The synthesis of branched-chain alkanes, such as isobutane (C4H10), used as a gasoline additive and refrigerant.
Polymerization: The formation of polymers, such as polyethylene (C2H4)n, from alkenes through free radical polymerization.
Free radical substitution in alkanes is a fundamental reaction mechanism in organic chemistry, providing a versatile approach for modifying the structure and properties of alkanes. Understanding the mechanism, factors affecting selectivity, and applications of free radical substitution is essential for chemists to design and control organic synthesis reactions and develop valuable products for various industries.